Own Brand Labelling -Medical Devices
In today's global market of medical devices , Own Brand Labelling has become a strategic approach and means for the distributors and healthcare brands to expand their reach without investment in manufacturing infrastructure.
Own Brand Labelling permits a company to market the products under their brand while the actual manufacturing is handled by the certified manufacturer , known as Original Equipment Manufacturer. This process aids the business in branding, distribution and customer relationships while the actual manufacturer for the production , quality control and regulatory compliance. The oem’s name may or may not appear on the medical device label depending on the distribution agreement and regulatory path.
Key Differences between OEM VS OBL
Aspects | OEM | OBL |
Role | Designing, Developing and Manufacturing the medical device | Marketing and Selling the medical device under their name |
Responsibility | Product Design , Testing, Certification, and CE , CDSCO Compliance | Branding, Labelling, Packaging and Distribution |
Regulation | Full Compliance with EU MDR CE, CDSCO | Relies on OEM’S technical data |
Documentation Access | Holds the technical file and conformity documents | Requires access to OEM’S technical documentation |
Brand Identity | The medical device carries oem’s name and logo | The medical device is labelled and marketed under the OBL company name. |
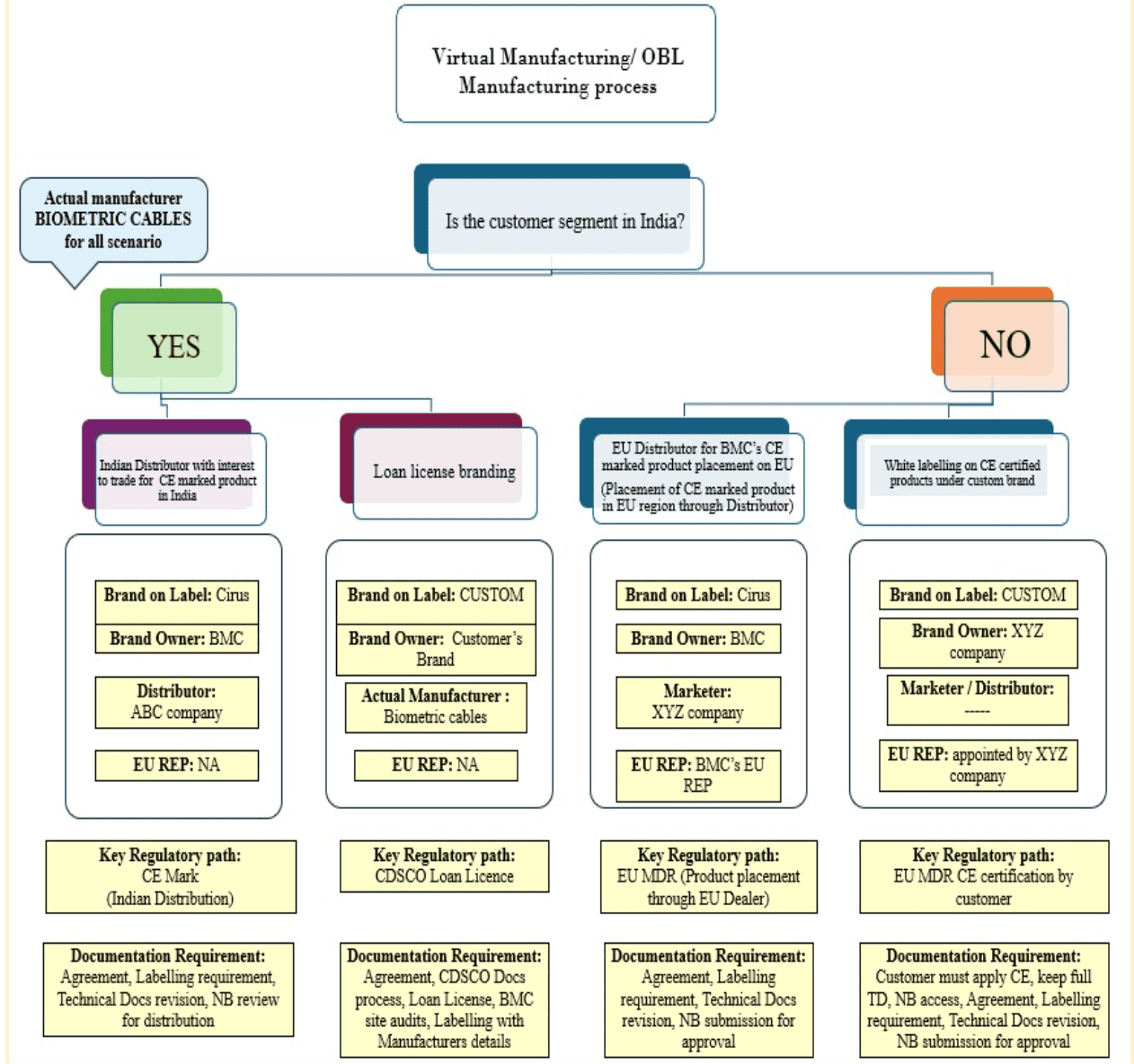
Own Branding Labelling Scenarios : How does it work in practice ?
EU Distributor with Exclusive Rights
Customer Segment | Manufacturer | Distributor | Brand on Label | Regulation Path | Documentation |
Outside India (EU Market) | Biometric Cables | ABC Company (Marketer) | Cirus (Example) | EU MDR with EU Representative | Label/Technical Documentation review and submission for NB approval |
When a distributor places Biometric Cables’ CE-marked product on the EU market, they assume the role of a legal distributor under EU MDR.
This means label and technical documents are revised to reflect the correct legal manufacturer and distributor details.
Indian Distributor for CE-Marked Products
Customer Segment | Manufacturer | Distributor | Brand on Label | Regulation Path | Documentation |
India | Biometric Cables | XYZ Company | Cirus (Example) | CE Mark for Indian Distribution | Label/Technical Documentation revision and NB review |
In India, CE marking is not mandatory under Indian MDR 2017, but it adds credibility.
When a CE mark is displayed, the distributor’s name and address must appear separately on the label, ensuring compliance with Notified Body (NB) review and approval.
3. EU Manufacturer White-Labelling CE-Marked Products
Customer Segment | Actual Manufacturer | Legal Manufacturer (Brand owner) | Brand on Label | Regulation Path | Documentation |
Outside India (EU) | Biometric Cables | ABC Company | Custom Brand (e.g., ABC) | MDR CE Certification by Customer | Full Technical Documentation access required |
Here, Biometric Cables acts as a subcontractor, while ABC becomes the legal manufacturer.
ABC company must obtain CE certification per EU MDR using Biometric Cables’ validated documentation.
Neutral coding can be used to mask manufacturer details, with CDSCO approval.
4. Indian Loan Licence Manufacturing
Customer Segment | Manufacturer | Distributor | Brand on Label | Regulation Path | Documentation |
India | Biometric Cables | XYZ Company | Custom Brand (Distributor’s name) | CDSCO Loan Licence under IMDR 2017 | CDSCO process, BMC site audits, and Loan Licence approvals |
The Loan Licence holder becomes the legal manufacturer under CDSCO, referencing Biometric Cables’ valid licence.
The product label may either mention the actual manufacturer or use a neutral code (with prior CDSCO approval).
This enables Indian distributors to market certified devices under their own brand while maintaining full regulatory compliance.
Benefits of Own Brand Labelling for Medical Devices Companies:
Launch the certified medical devices under your own brand name without the lengthy process of R&D, product testing, or Manufacturing. The OBL model significantly accelerates your time-to-market.
Save on costly product development and certification expenses by utilizing the OEM’s already CE-marked products and approved technical documentation.
OBL partners benefit from ready access to validated technical documentation and MDR/CE conformity data provided by the OEM, ensuring seamless compliance with EU MDR, CDSCO, and other regional regulatory requirements.
Partnering with a compliant OEM allows your brand to expand into multiple markets including the EU, Middle East, Asia, and India all under a unified certification framework.
Tailor product labels, designs, and packaging to reflect your brand identity while adhering to strict technical and safety compliance standards.
OEMs maintain full control over design and production data while granting OBL partners limited access to technical documentation for regulatory review ensuring complete protection of intellectual property.
Enhance credibility and brand recognition within the medical community by showcasing your name on premium, certified medical devices.
As market demand increases, OBL partnerships enable rapid, seamless, and sustainable scaling — eliminating the need for additional certifications or factory expansions.
Who can get benefitted from the OBL model ?
Medical device distributors
Emerging Healthcare Companies
Hospital and Healthcare Networks
Exporters and Regional Distributors
E-Commerce and Regional Healthcare Platforms
Medical device manufacturers for Accessory requirements.
European regulatory Updates: Virtual Manufacturing
The Commission Recommendation 2013/473/EU, issued by the European Commission on 24 September 2013, sets out clear guidance for how Notified Bodies should conduct audits and assessments of medical device manufacturers to ensure product safety, quality, and regulatory compliance.
It was introduced to strengthen oversight following inconsistent auditing practices across EU Member States and incidents involving non-compliant devices.
The European Union Medical Device Regulation (EU MDR 2017/745) has made OBL increasingly difficult. Under the new MDR framework, OBL companies can no longer rely solely on an OEM’s CE certificate. They must hold their own technical documentation and regulatory accountability.
This shift has paved the way for Virtual Manufacturing(Formerly Own brand labelling), where the marketing company assumes the role of the legal manufacturer while outsourcing production to an MDR-compliant OEM. This ensures full regulatory independence, data transparency, and market sustainability.
Indian Medical Device Regulatory Updates
In India, many medical device companies want to market their products under their own brand name but lack manufacturing facilities. The good news? You can still launch certified, compliant devices through the Loan Licence model, recognized under the Medical Device Rules (MDR) 2017 and regulated by the Central Drugs Standard Control Organisation (CDSCO).
A Loan Licence enables an Own Brand Labelling (OBL) company to produce medical devices through a licensed OEM’s approved facility.
Under this arrangement:
The OBL partner (loan licensee) is recognized by CDSCO as the legal manufacturer.
Production takes place at the OEM’s authorized manufacturing site under a formal agreement between both parties.
The loan licence holder bears complete regulatory accountability covering product compliance, labeling, and post-market vigilance.
This model is commonly adopted by startups, distributors, and private-label healthcare companies aiming to broaden their product range without establishing their own manufacturing units.
OBL companies can achieve faster market entry by utilizing compliant OEM facilities, avoiding the lengthy setup and certification processes typically required for new manufacturers. They also benefit from the OEM’s established ISO 13485-certified Quality Management System, as well as validated Plant Master File (PMF) and Device Master File (DMF), which streamline loan licence approvals under CDSCO. This model significantly reduces capital expenditure, allowing companies to avoid investments in manufacturing infrastructure while still maintaining regulatory credibility and full brand ownership.
Under MDR 2017, labeling must clearly display both the Loan Licence Holder (OBL brand) and the OEM facility details, including the address and license number. The loan licence route is particularly advantageous for startups and small-to-medium enterprises seeking to expand their branded product portfolios without setting up their own manufacturing units, making it an efficient and scalable pathway for growth in the healthcare sector.
Regulatory Process for Loan Licence under CDSCO:
Application Forms:
Class C & D devices: Apply with Form MD-8 and receive Form MD-10 upon approval.
Class A & B devices: Managed by State Licensing Authority through Form MD-4 and approval process.
Key Documentation Required:
Cover letter & constitution of firm
Fee challan and ownership/tenancy agreement
Formal agreement between OBL and OEM
OEM’s manufacturing licence
PMF & DMF copies
QMS compliance certificate
Labelling samples, risk analysis, validation data
Post-Market Surveillance (PMS) reports
Batch release certificates
Inspection Process:
Class A devices: No pre-audit required (for non-sterile, non-measuring devices).
Class B, C & D devices: Mandatory facility audit by CDSCO prior to licence approval.
Legal Responsibility:
The loan licence holder remains fully responsible for regulatory compliance, vigilance, and product safety, even though physical manufacturing is handled by the OEM.
Manufacturing License vs Loan License :
Aspects | Manufacturing License | Loan License |
User | Direct Manufacturers | Used by OBL Companies |
Facility Ownership | Owned by Applicant | OEM facility used |
Regulatory Responsibility | Manufacturer | Loan License Holder |
Key Forms | MD 5/MD 9 | MD-8, MD-10 |
Labelling requirement | Manufacturer only | OEM +OBL Site address |
Typical Usecase | Large-scale producers | Startups, SMEs, private labels |

